All Weeks Introduction to battery-management systems Coursera Quiz Answers
Table of Contents
Introduction to battery-management Systems Week 01 Quiz Answers
Practice quiz for lesson 1.1.1 Quiz Answers
Q1. Based on the definition in this lesson, which of the following is not an “embedded system”?
- A computer printer
- A scientific calculator
- A home security system
- A digital camera
- A desktop personal computer (PC)
Q2. Which of the following are the functions of a battery-management system (BMS)? Select all that apply.
- Prolong the life of the battery
- Protect the safety of human operators of battery-powered application
- Protect the cells of the battery from damage
- Provide the battery-powered application with guidance regarding how to make the best use of the battery pack
- Control the rate at which the battery pack is discharged while it is being used
Q3. Which of the following battery-powered applications might not need a BMS?
- The lead-acid 12V system in an automobile
- The lithium-ion battery pack in an electric vehicle
- A lithium-ion battery pack for domestic power backup
- The lithium-ion battery pack in a hybrid-electric vehicle
- A lithium-ion battery pack for energy storage for the utility grid
Practice quiz for lesson 1.1.2 Quiz Answers
Q1. Consider the mobile-phone lithium-ion battery cell shown below (both sides are shown).

Based on the markings on this cell, state the cell’s nominal voltage (in volts).
- 3.8- Nominal Voltage
Q2. Consider the mobile-phone lithium-ion battery cell shown below (both sides are shown).
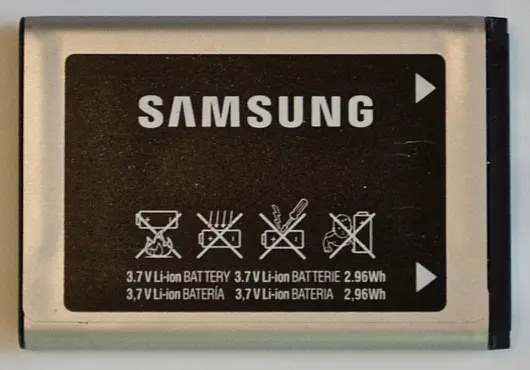

What is the 1C rate for this cell (in mA)?
- 800- 1C rate
Q3. Consider the mobile-phone lithium-ion battery cell shown below.

What is the nominal energy storage capacity of this cell (in Wh)? Enter your answer with two digits to the right of the decimal point.
- 6.66- NESC
Q4. Consider the mobile-phone lithium-ion battery cell shown below (both sides are shown).

if ten of these cells are connected in series to create a battery, what is the nominal voltage of the battery (in V)?
Comment down Answers if you found
Q5. Consider the mobile-phone lithium-ion battery cell shown below.
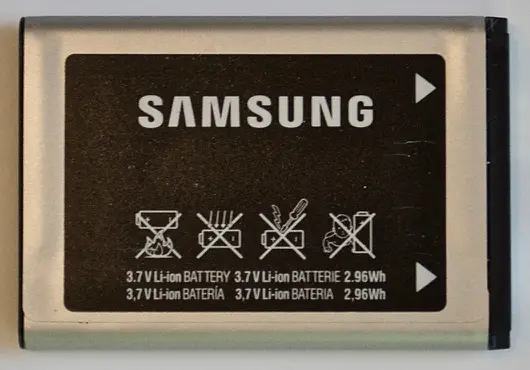
if two of these cells are connected in parallel to create a battery, what is the nominal energy capacity of the battery (in Wh)? Please enter your answer with two digits following the decimal point.
- Comment down Answers if you found
Practice quiz for lesson 1.1.3 Quiz Answers
Q1. In which electrode does oxidation occur when a cell is discharged? (Enter “negative” or “positive”, without the quotes.)
- negative
Q2. If the net movement of cations through the electrolyte of an electrochemical cell is from the positive electrode region toward the negative electrode region, is the cell being charged or discharged?
- Charged.
- Discharged.
Q3. Which component(s) of a cell must be designed to prevent self-discharge? (Select all that apply.)
- The electrolyte
- The separator
- The negative electrode
- The current collectors
- The positive electrode
Practice quiz for lesson 1.1.4 Quiz Answers
Q1. A battery cell stores energy as:
- Gravitational potential energy
- Electric kinetic energy
- Electromagnetic energy
- Electrochemical potential energy
- Nuclear potential energy
Q2. In every battery cell that is not completely discharged (select all that apply):
- The potential energy at the negative electrode favors a process that releases electrons into the external circuit and cations into the electrolyte
- The potential energy at the positive electrode favors a process that accepts electrons from the external circuit and releases cations into the electrolyte
- The potential difference between the cell’s electrodes produces an externally measurable quantity known as cell voltage
- The cell can be connected to an external circuit, converting stored energy into electrical kinetic energy
- The cell can be recharged to a full state of charge by injecting energy into the cell
Q3. When a battery cell is being charged, you observe that the charging current appears to be constant for a period of time while the voltage is increasing, and then the magnitude of the charging current decreases until it is essentially zero while the voltage is kept constant. Select all that apply:
- This type of charging profile is used to ensure that manufacturer-specified voltage limits are not violated, in order to promote safety.
- The battery cell is fully charged after the first period of time when the voltage has reached its maximum value.
- This battery cell has undergone a constant-current constant-voltage charge profile.
- The battery cell has undergone a constant-power-voltage charge profile.
- The battery cell’s state of charge increases over the entire interval.
Practice quiz for lesson 1.1.5 Quiz Answers
Q1. What is the mean atomic weight of lithium? (Enter three digits to the right of the decimal point.)
- The mean atomic weight of lithium is approximately 6.941.
Q2. How many valence electrons does carbon have?
- Carbon has 4 valence electrons.
Q3. Consider an electrochemical cell where the negative electrode is sodium (Na) and the positive electrode is copper (Cu). What would be the standard potential of this cell? (Enter two digits to the right of the decimal point.)
- The standard potential of the cell with sodium (Na) as the negative electrode and copper (Cu) as the positive electrode would be approximately 2.71 volts.
Quiz for Week 1 Quiz Answers
Q1. Which of the following are examples of automotive embedded systems (select all that apply)?
- The traction-control system
- The engine controller
- The manual hand brake
- The stability-control system
- The air-bag controller
Q2. Which of the following is usually not a function performed by a battery management system (BMS)?
- Maintain the battery in a state in which it can fulfill its functional design requirements
- Protect the safety of the human operator of the battery-powered application
- Enforcing the maximum power an application can draw from the battery pack
- Control the battery-pack charger
- Protect the cells of the battery pack from damage
Q3. When an electrochemical cell is being discharged, which cell component gives up electrons to the external circuit?
- The negative electrode
- The positive electrode
- The electrolyte
- The separator
- The current collectors
Q4. Which of the following statements is true?
- During discharge, anions move through the electrolyte toward the negative electrode.
- During discharge, cations move through the electrolyte toward the negative electrode.
- During discharge, the positive electrode provides electrons to the external circuit.
- During charge, the negative electrode is oxidized
- During discharge, the separator prevents ions in the electrolyte from flowing from one electrode region to the other
Q5. When a battery cell is being charged, you observe that charging power appears to be constant for a period of time while voltage is increasing, and then the magnitude of charging power decreases until it is essentially zero while voltage is kept constant. Select all that apply:
- The battery cell has undergone a constant-power-voltage charge profile.
- This type of charging profile is used to ensure that manufacturer-specified voltage limits are not violated, in order to promote safety.
- The battery cell is fully charged after the first period of time when the voltage has reached its maximum value.
- This battery cell has undergone a constant-current constant-voltage charge profile.
- The battery cell’s state of charge increases over the entire interval.
Q6. Consider an electrochemical cell where the negative electrode is zinc (Zn) and the positive electrode is copper (Cu). What would be the standard potential of this cell? (Enter one digit to the right of the decimal point.)
- The standard potential of the cell with zinc (Zn) as the negative electrode and copper (Cu) as the positive electrode would be approximately 0.34 volts.
Q7. Which of the following elements are alkali metals? (Select all that apply.)
- Sodium
- Potassium
- Bromine
- Chlorine
- Fluorine
- Lithium
Introduction to battery-management Systems Week 02 Quiz Answers
Practice quiz for lesson 1.2.1 Quiz Answers
Q1. Which of the following is true?
- For a given storage capacity, lower specific energy cells are lighter.
- For a given volume, lower energy density stores more energy.
- For a given storage capacity, higher energy density cells are larger.
- For a given weight, higher specific energy stores more energy.
Q2. Which of the following are the advantages (s) of lithium-ion cells versus most other secondary cells? Select all that apply.
- They have higher energy density.
- They have higher nominal voltages.
- They have lower self-discharge rates.
- They are simpler to manufacture.
Q3. Which of the following are the disadvantages (s) of lithium-ion cells versus most other secondary cells? Select all that apply.
- They require additional safety precautions because their chemistry is not as stable as others.
- They have shorter expected life.
- They are more expensive than similar-capacity cells having different chemistry.
- They are more complex to manufacture.
Practice quiz for lesson 1.2.2 Quiz Answers
Q1. Which of the following statements are true concerning intercalation compounds? Select all that apply.
- Lithium moves automatically from high-concentration regions of the compound to low-concentration regions of the compound via diffusion
- The compound must have an open crystal structure, allowing insertion or extraction of lithium ions in the vacant spaces
- Lithium forms a strong chemical bond with the compound when it intercalates into its crystal structure
- When Li^++ enters the compound, the compound also must have the ability to accept compensating electrons from the external circuit
Q2. In addition to the electrode materials themselves, what else might we expect to find in the positive-electrode region of a lithium-ion cell? Select all that apply.
- Electrolyte.
- Binders.
- Conductive additives.
- Electrolyte additives.
Q3. When discharging a lithium-ion cell, which of the following is true? Select all that apply.
- Lithium diffuses from the center of a negative-electrode particle toward its surface
- Lithium diffuses from the center of a positive-electrode particle toward its surface
- Lithium at the surface of negative-electrode particles gives up electrons and enters the electrolyte as Li^++ cations
- Lithium at the surface of positive-electrode particles gives up electrons and enters the electrolyte as Li^++ cations
- Li^++ cations diffuse through the electrolyte from high-concentration regions to low-concentration regions
Practice quiz for lesson 1.2.3 Quiz Answers
Q1. Which of the following is NOT a good candidate material to use in a lithium-ion negative electrode?
- Graphite
- Silicon
- Hard carbon
- Lithium cobalt oxide (LiCoO_22, or “LCO”)
- Lithium titanate oxide (Li_44Ti_55O_{12}12, or “LTO”)
Q2. Which of the following statements is true regarding cells having lithium titanate oxide (Li_44Ti_55O_{12}12, or “LTO”) negative electrodes?
- LTO cells can have extremely long lifetimes when compared with lithium-ion cells having different negative-electrode materials.
- LTO cells are inexpensive to manufacture compared with lithium-ion cells and have different negative-electrode materials.
- LTO cells have higher energy density than lithium-ion cells having different negative-electrode materials.
- LTO cells have higher per-cell open-circuit voltage than lithium-ion cells having different negative-electrode materials.
Q3. Which of the following is true regarding cells having silicon or silicon-containing negative electrodes?
- The energy density of cells having silicon-negative electrodes can be very high
- The life of cells having silicon-negative electrodes is usually very high
- Electrode volume changes are minimized when using silicon or silicon-containing negative electrodes
- Most lithium-ion cells presently used in applications have silicon or silicon-containing negative electrodes
Practice quiz for lesson 1.2.4 Quiz Answers
Q1. Which of the following are “layered” positive-electrode materials? Select all that apply.
- Lithium iron phosphate (Li_xxFePO_44, or “LFP”)
- Lithium cobalt oxide (Li_xxCoO_22, or “LCO”)
- Lithium nickel manganese cobalt oxide (“NMC”)
- Lithium manganese oxide (Li_xxMn_22O_44, or “LMO”)
- Lithium nickel cobalt aluminum oxide (“NCA”)
Q2. Which of the following statements are true regarding a comparison between NMC and LCO? Select all that apply.
- Replacing some cobalt with manganese increases toxicity
- Replacing some cobalt with nickel increases cell safety
- Replacing some cobalt with nickel increases cell voltage
- Replacing some cobalt with manganese decreases the cost
Q3. Which of the following statements is not true regarding material structure?
- 1D structures tend to have the least resistance, 3D structures tend to have the highest resistance, and 2D structures tend to have intermediate resistance.
- Layered materials can use only about half of their theoretic capacity reversibly.
- Olivine materials have low voltage and their voltage contains very little state information
- Spinel materials are inexpensive and non-toxic but can degrade rapidly
Practice quiz for lesson 1.2.5 Quiz Answers
Q1. What are some key properties that must be satisfied by an electrolyte for a lithium-ion cell? (Select all that apply.)
- It must be aqueous.
- It must comprise a solvent plus a lithium-based salt.
- It must be able to withstand voltages in excess of 3V without breaking down.
- It must conduct ions with little resistance but be an electronic insulator.
Q2. Desirable properties for separators in lithium-ion cells include: (select all that apply)
- The separator must be an electronic insulator.
- The separator must have pores through which ions in the electrolyte can move to traverse from one side to the other.
- The separator must have holes large enough for negative-electrode particles to contact positive-electrode particles to complete an electrical circuit.
- The separator must be as thick as budget constraints allow.
Q3. The negative-electrode current collector is usually made from a metal foil of which element?
- Cu
Practice quiz for lesson 1.2.6 Quiz Answers
Q1. Which of the elements in the following list is more abundant than the others, according to the U.S. Geological Survey?
- Lithium
- Lead
- Nickel
- Cadmium
Q2. Lithium content in a high-energy lithium-ion cell is about what percentage, by weight?
- About 3%
- About 10%
- About 25%
- About 50%
Q3. If we were able to harvest all of the lithium on the planet and use it to make battery packs for electric vehicles, approximately how many electric vehicles could each human being presently own (without recycling)?
- About 2000.
- About 1.
- About 5.
- About 100.
- About 1000.
Quiz for Week 2 Quiz Answers
Q1. Which of the following is true?
- For a given storage capacity, higher energy density cells are larger.
- For a given weight, lower specific energy stores more energy.
- For a given storage capacity, lower specific energy cells are lighter.
- For a given volume, higher energy density stores more energy.
Q2. Which of the following statements are true concerning intercalation compounds? Select all that apply.
- Lithium moves automatically from high-concentration regions of the compound to low-concentration regions of the compound via diffusion
- When Li^++ enters the compound, the compound also must have the ability to accept compensating electrons from the external circuit
- Lithium forms a strong chemical bond with the compound when it intercalates into its crystal structure
- The compound must have an open crystal structure, allowing insertion or extraction of lithium ions in the vacant spaces
Q3. When charging a lithium-ion cell, which of the following are true? Select all that apply.
- Lithium at the surface of negative-electrode particles gives up electrons and enter the electrolyte as Li^++ cations
- Lithium at the surface of positive-electrode particles give up electrons and enters the electrolyte as Li^++ cations
- Lithium diffuses from the center of a positive-electrode particle toward its surface
- Li^++ cations diffuse through the electrolyte from high-concentration regions to low-concentration regions
- Lithium diffuses from the center of a negative-electrode particle toward its surface
Q4. Which of the following is not a good candidate material to use in a lithium-ion negative electrode?
- Silicon
- Hard carbon
- Lithium titanate oxide (Li_44Ti_55O_{12}12, or “LTO”)
- Graphite
- Lithium cobalt oxide (LiCoO_22, or “LCO”)
Q5. Which of the following statements is true regarding cells having lithium titanate oxide (Li_44Ti_55O_{12}12, or “LTO”) negative electrodes?
- LTO cells are inexpensive to manufacture compared with lithium-ion cells having different negative-electrode materials.
- LTO cells have higher energy density than lithium-ion cells having different negative-electrode materials.
- LTO cells tend to have short lifetimes when compared with lithium-ion cells having different negative-electrode materials.
- LTO cells have lower per-cell open-circuit voltage than lithium-ion cells having different negative-electrode materials.
Q6. Which of the following are “cubic-spinel” positive-electrode materials? Select all that apply.
- Lithium iron phosphate (Li_xxFePO_44, or “LFP”)
- Lithium cobalt oxide (Li_xxCoO_22, or “LCO”)
- Lithium manganese oxide (Li_xxMn_22O_44, or “LMO”)
- Lithium nickel manganese cobalt oxide (“NMC”)
- Lithium nickel cobalt aluminum oxide (“NCA”)
Q7. Which of the following statements are true regarding a comparison between NMC and LCO? Select all that apply.
- Replacing some cobalt with manganese increases toxicity
- Replacing some cobalt with manganese decreases cost
- Replacing some cobalt with nickel increases cell voltage
- Replacing some cobalt with nickel increases cell safety
Q8. What are some key properties that must be satisfied by an electrolyte for a lithium-ion cell? (Select all that apply.)
- It must be able to withstand voltages in excess of 3V without breaking down.
- It must be aqueous.
- It must conduct ions with little resistance but be an electronic insulator.
- It must comprise a solvent plus a lithium-based salt.
Q9. The negative-electrode current collector is usually made from a metal foil of which element?
- The negative-electrode current collector is usually made from a metal foil of the element copper (Cu).
Q10. Which of the elements in the following list is more abundant than the others, according to the U.S. Geological Survey?
- Nickel
- Cadmium
- Lead
- Lithium
Introduction to battery-management Systems Week 03 Quiz Answers
Practice quiz for lesson 1.3.1 Quiz Answers
Q1. Which of the following is needed in order for a BMS to fulfill its priority to “protect the safety of the operator of the host application”? Select all that apply.
- Detect the presence of a ground fault
- Control the thermal management system
- Estimating the state-of-life (SOL) of the battery cells in the battery pack
- Measure all cell voltages, pack current, and temperature at different locations in the battery pack
Q2. Which of the following tasks fall under “BMS Requirement 1: Sensing and high-voltage control”? Select all that apply.
- Measuring battery-pack electrical current
- Measuring the voltage of all the cells in the battery pack
- Measuring total charge capacities of all cells in the battery pack
- Measuring the state of charge of all cells in the battery pack
- Measuring temperature at different points in the battery pack
Q3. Why might we choose not to implement all of the possible features in a particular BMS application?
- Because the battery is inexpensive enough that the extra cost of additional BMS functionality is not justified
- Because it is too difficult to design and build the electronics of a BMS that implements all of the features described in this specialization
- Because it is too difficult to design and implement the software in a BMS that implements all of the features described in this specialization
- Because the battery is already so expensive that the extra cost of additional BMS functionality does not fit in the budget
Practice quiz for lesson 1.3.2 Quiz Answers
Q1. What is the nominal energy capacity (in kWh) of a 1P50S battery pack built from lithium-ion cells each having a nominal capacity of 10Ah, a nominal voltage of 3.8V, and a present current-carrying capability of 20A??
- 1.9kWh
Q2. In a 4P100S battery pack built using series-cell modules (SCMs), how many cells are there in each module?
- 4
Q3. In a master/slave BMS design, which of the following are usually functions performed by the slaves? Select all that apply.
- Measure the electrical current passing through the cells in the module to which it is connected
- Measure voltage of every cell within the module to which it is connected
- Balance the energy stored in every cell within the module to which it is connected
- Measure one or more temperatures representative of the cells in the module to which it is connected
Practice quiz for lesson 1.3.3 Quiz Answers
Q1. If an analog-to-digital converter has an input range of 0V through 5V, and has 10 bits of precision, what is the resolution of the converter (in mV)? Round your answer to the nearest mV.
The resolution of an analog-to-digital converter (ADC) with 10 bits of precision and an input range of 0V through 5V can be calculated as follows:
Resolution (in mV) = (Input range (in V)) / (2^Number of bits)
Resolution (in mV) = (5V) / (2^10) = 5V / 1024 ≈ 4.88 mV
Rounded to the nearest mV, the resolution is approximately 5 mV.
Q2. If an analog-to-digital converter has an input range of 0V through 5V, has 12 bits of precision, what is the maximum quantization error possible for the converter (in \muμV)? Round your answer to the nearest \muμV.
The maximum quantization error (in μV) for an ADC with 12 bits of precision and an input range of 0V through 5V can be calculated as follows:
Maximum Quantization Error (in μV) = (Input range (in V)) / (2^(Number of bits – 1))
Maximum Quantization Error (in μV) = (5V) / (2^(12 – 1)) = 5000 μV / 2048 ≈ 2.44 μV
Rounded to the nearest μV, the maximum quantization error is approximately 2 μV.
Q3. What are some features that are commonly implemented on BMS slave chipsets? Select all that apply.
- The ability to connect multiple instances of the same integrated circuit in parallel for redundant fault-tolerant designs
- High-precision, high-accuracy isolated sensing of battery-pack electrical current
- Temperature sensing at one or more points in a module of series-connected battery cells
- Voltage sensing with high common-mode rejection and fast response in high-EMI, high-heat, high-vibration environments
Practice quiz for lesson 1.3.5 Quiz answers
Q1. Consider a 0.1m\OmegaΩ current-sensor shunt, connected via an amplifier with gain factor of 100 to an analog-to-digital converter. If the measured voltage is 1V, what amount of electrical current is passing through the shunt (in A)? Round your answer to the nearest A.
100 Amp.
First calculate the initial voltage without amplification i.e. V(unamplified)= 1V/(100 (i.e. gain factor)= 0.01V.
Now, use this voltage to calculate current, I= V/R, I= 0.01/0.0001=> 100 A
Q2. What is the primary advantage to using a current-sensor shunt instead of a Hall-effect sensor?
- Shunts have no measurement offset at zero current, so are good for avoiding drift in coulomb counting
- Current-shunt sensors are electrically isolated from the battery pack, so no special isolation circuitry is needed
- Shunt measurements are not affected by temperature, and so it is simple to use shunts for battery systems that must operate over wide temperature ranges
- The shunt-sensor voltage can be measured directly by the battery-management system without specially designed circuitry
Q3. What is the primary advantage to using a Hall-effect current sensor instead of a shunt current sensor?
- Hall-effect sensors do not require a four-wire Kelvin connection in order to measure battery-pack current
- Hall-effect sensors are electrically isolated from pack current, so no special isolation circuitry is needed
- Hall-effect sensors have no measurement offset at zero current, so are good for avoiding drift in coulomb counting
- Hall-effect sensors are more precise than shunt current sensors
Practice quiz for lesson 1.3.6 Quiz Answers
Q1. Which of the following statements are true about contactors and their use in BMS? Select all that apply.
- A contractor uses a low-voltage low-power input to switch a high-voltage high-power current pathway
- A precharge contactor is needed to prevent damage to the main contactor(s) when connecting to a capacitive load
- Contactors are needed to protect the battery pack against the internal short circuit of one or more of its battery cells
- High-voltage high-power battery-pack designs most often employ three separate contractors
Q2. When connecting a battery pack to its load, which of the following sequences is listed in the correct order?
- 1) Connect one battery terminal to the load
- 2) Connect the other battery terminal to the load through the precharge circuit
- 3) Connect the second battery terminal to the load
- 4) Disconnect the precharge circuit
Q3. When connecting a battery pack to a load, when should the precharge circuit be deactivated? Select all that apply.
- When the difference between battery-pack voltage and bus voltage drops below some small design threshold
- When the electrical current flowing through the battery pack drops below some small design threshold
- When the temperature of the precharge resistance exceeds some design threshold
- After the precharge circuit has been activated for a specified period of time
Practice quiz for lesson 1.3.7 Quiz Answers
Q1. Consider a battery having V_b=100Vb=100V. What is the minimum value of isolation resistance (in k\OmegaΩ) for the battery pack to be considered safe?
- The minimum value of isolation resistance for a battery pack to be considered safe depends on various factors, including the voltage level, safety standards, and specific application requirements. In general, a higher voltage battery pack would require a higher isolation resistance to ensure safety. However, a common guideline is to have an isolation resistance of at least 1 MΩ (megaohm) for every 100 volts of battery voltage. So, for a 100V battery pack, a minimum isolation resistance of 1 MΩ is often considered safe. Please note that actual safety standards and requirements may vary, so it’s essential to consult relevant standards and guidelines for your specific application.
Q2. Consider a battery having V_b=200Vb=200V. We measure V_1=50V1=50V and V_2=150V2=150V. If we insert a known resistance R_0=200R0=200k\OmegaΩ on the negative-terminal side of the battery, then we measure V_1’=48.3V1′=48.3V. If, instead, we insert a known resistance R_0=200R0=200k\OmegaΩ on the positive-terminal side of the battery, then we measure V_2’=145V2′=145V.
What is the isolation resistance of this battery pack (in \OmegaΩ)? Round your answer to the nearest \OmegaΩ.
Answer:
To calculate the isolation resistance of the battery pack, we can use the formula:
Isolation Resistance (R_iso) = (R_0 * (V_1 – V_1′)) / (V_1′ – V_2)
Substituting the given values:
R_0 = 200 kΩ V_1 = 50 V V_1′ = 48.3 V V_2 = 150 V
R_iso = (200,000 Ω * (50 V – 48.3 V)) / (48.3 V – 150 V) R_iso = (200,000 Ω * 1.7 V) / (-101.7 V) R_iso ≈ -3,405,882.35 Ω
The calculated isolation resistance is approximately -3,405,882.35 Ω. Please double-check the values provided or the calculation formula, as negative resistance doesn’t have a physical meaning in this context. It’s possible there may be an error in the provided data or calculations.
Q3. Consider a battery having V_b=150Vb=150V. We measure V_1=80V1=80V and V_2=70V2=70V. If we insert a known resistance R_0=100R0=100k\OmegaΩ on the negative-terminal side of the battery, then we measure V_1’=40V1′=40V. If, instead, we insert a known resistance R_0=100R0=100k\OmegaΩ on the positive-terminal side of the battery, then we measure V_2’=35V2′=35V.
What is the isolation resistance of this battery pack (in \OmegaΩ)? Round your answer to the nearest \OmegaΩ.
Answer:
To calculate the isolation resistance of the battery pack, we can use the formula:
Isolation Resistance (R_iso) = (R_0 * (V_1 – V_1′)) / (V_1′ – V_2)
Substituting the given values:
R_0 = 100 kΩ V_1 = 80 V V_1′ = 40 V V_2 = 70 V
R_iso = (100,000 Ω * (80 V – 40 V)) / (40 V – 70 V) R_iso = (100,000 Ω * 40 V) / (-30 V) R_iso ≈ -133,333.33 Ω
The calculated isolation resistance is approximately -133,333.33 Ω. Please double-check the values provided or the calculation formula, as negative resistance doesn’t have a physical meaning in this context. It’s possible there may be an error in the provided data or calculations.
Quiz for Week 3 Quiz Answers
Q1. What is the power capability (in kW) of a 1P50S battery pack built from lithium-ion cells each having a nominal capacity of 10Ah, a nominal voltage of 3.8V, and a present current-carrying capability of 100A?
- power capability of the battery pack is 10,000 kW.
Q2. In a 6P120S battery pack built using series-cell modules (SCMs), how many cells are there in each module?
- 120
Q3. In a master/slave BMS design, which of the following are usually functions performed by the slaves? Select all that apply.
- Measure the electrical current passing through the cells in the module to which it is connected
- Measure the voltage of every cell within the module to which it is connected
- Measure one or more temperatures representative of the cells in the module to which it is connected
- Balance the energy stored in every cell within the module to which it is connected
Q4. If an analog-to-digital converter has an input range of 0V through 5V, and has 12 bits of precision, what is the resolution of the converter (in mV)? Round your answer to the nearest mV.
- 1.22mv
Q5. If an analog-to-digital converter has an input range of 0V through 5V, has 10 bits of precision, what is the maximum quantization error possible for the converter (in mV)? Round your answer to the nearest mV.
- 4.88mv
Q6. Consider the voltage-divider circuit:
What is the voltage vv (in V)?
- 3.33mv
Q7. Consider an NTC thermistor having R_0 = 100R0=100k\OmegaΩ, T_0 = 25^\circT0=25∘C, and \beta=4282β=4282 connected in a voltage divider circuit with R_1=100R1=100k\OmegaΩ and v=5v=5V. If we measure v_{therm}=2vtherm=2V, what is the temperature of the thermistor (in ^\circ∘C)? Round your answer to the nearest ^\circ∘C.
- 34
Remember that the thermistor equation is: R_{therm} = R_0 \exp\left(\beta\left(\frac{1}{273.15+T} – \frac{1}{273.15+T_0}\right)\right)Rtherm=R0exp(β(273.15+T1−273.15+T01)).
Q8. Consider a 0.1m\OmegaΩ current-sensor shunt, connected via an amplifier with gain factor of 200 to an analog-to-digital converter. If the measured voltage is 4V, what amount of electrical current is passing through the shunt (in A)? Round your answer to the nearest A.
- 200
Q9. What is the primary advantage to using a current-sensor shunt instead of a Hall-effect sensor?
- Shunt measurements are not affected by temperature, and so it is simple to use shunts for battery systems that must operate over wide temperature ranges
- The shunt-sensor voltage can be measured directly by the battery-management system without specially designed circuitry
- Current-shunt sensors are electrically isolated from the battery pack, so no special isolation circuitry is needed
- Shunts have no measurement offset at zero current, so are good for avoiding drift in coulomb counting
Q10. When connecting a battery pack to its load, which of the following sequences is listed in the correct order?
- Connect one battery terminal to the load
- Connect the other battery terminal to the load through the precharge circuit
- Connect the second battery terminal to the load
- Disconnect the precharge circuit
- Connect one battery terminal to the load
- Connect the second battery terminal to the load
- Connect the other battery terminal to the load through the precharge circuit
- Disconnect the precharge circuit
- Connect one battery terminal to the load through the precharge circuit
- Connect the same battery terminal to the load
- Disconnect the precharge circuit
- Connect the second battery terminal to the load
- Connect one battery terminal to the load
- Connect the same battery terminal to the load through the precharge circuit
- Connect the second battery terminal to the load
- Disconnect the precharge circuit
Q11. Consider a battery having V_b=350Vb=350V. What is the minimum value of isolation resistance (in k\OmegaΩ) for the battery pack to be considered safe (per the standard discussed in lesson 1.3.7)?
- 175k
Q12. Consider a battery having V_b=300Vb=300V. We measure V_1=160V1=160V and V_2=140V2=140V. If we insert a known resistance R_0=150R0=150k\OmegaΩ on the negative-terminal side of the battery, then we measure V_1’=80V1′=80V. If, instead, we insert a known resistance R_0=150R0=150k\OmegaΩ on the positive-terminal side of the battery, then we measure V_2’=70V2′=70V.
- 150k
Q13. What is the isolation resistance of this battery pack (in \OmegaΩ)? Round your answer to the nearest \OmegaΩ.
- The isolation resistance of this battery pack is 150Ω (rounded to the nearest Ω) for both cases.
Introduction to battery-management systems Week 04 Quiz Answers
Practice quiz for lesson 1.4.1 Quiz Answers
Q1. Which of the following detection mechanisms could protect a battery pack from overtemperature? Select all that apply.
- Thermal fuse
- Contactor
- Electronic protection
- Resettable fuse
Q2. Which of the following detection mechanisms could protect a battery pack from an out-of-tolerance voltage? Select all that apply.
- Thermal fuse
- Resettable fuse
- Electronic protection
- Contactor
Q3. What type(s) of fault(s) should a BMS be able to detect? Select all that apply.
- Communication faults
- Isolation faults
- Cell faults
- Processor faults
Practice quiz for lesson 1.4.2 Quiz Answers
Q1. For which of the following communication needs do you think that the high-speed CAN protocol must be used to communicate between the BMS and the host application instead of the low-speed protocol?
- For reporting detection of an emergency condition (e.g., a short-circuit fault in one of the cells) that requires the BMS to disconnect the battery pack from the loadFor reporting battery cell state of charge information to the host application
- For reporting battery cell state of health information to the host application
- For reporting vehicle range estimates to the host application
Q2. Suppose that an electric sport-utility passenger vehicle expends energy at a rate of 220Wh/km while driving. If this vehicle’s battery pack is charged at a 6.6kW rate, what is the rate of range added to the battery in “km (of range added to battery charge) per hour”? Round your answer to the nearest “km/h”
- 6.6kwh / 220 wh/km = 30km
Q3. Which of the following data would probably not be stored in the “log book” permanent memory of the BMS?
- An exhaustive record of computed available power versus time for the battery pack
- Out of tolerance voltage, current, and/or temperature
- Number of discharge/charge cycles completed
- Battery pack state-of-health when the BMS was last shut down or powered up
Practice quiz for lesson 1.4.3 Quiz Answers
Q1. Which battery-pack quantities might a host application need to know? Select all that apply.
- Temperature
- State of charge
- Remaining energy
- Available power
Q2. Which of the following quantities must we estimate because it is impossible to measure their values? Select all that apply.
- Cell voltage v_kvk
- Cell temperature T_kTk
- Cell total charge capacity Q_kQk
- Cell resistance R_kRk
- Cell state of charge z_kzk
Q3. What are some consequences of using a simple BMS power-limits algorithm instead of a more accurate but more complicated BMS algorithm?
- A bad estimate might lead to overcharging or over-discharging cells
- Abrupt corrections when voltage or current limits exceeded
- A larger BMS algorithm-design staff is needed
- A need to compensate for algorithm uncertainty by oversizing the battery pack
Practice quiz for lesson 1.4.4 Quiz Answers
Q1. Suppose that a negative-electrode material has \theta_{0\%}^{\rm neg}=0.01θ0%neg=0.01 and \theta_{100\%}^{\rm neg}=0.81θ100%neg=0.81. If \theta_k^{\rm neg}=0.61θkneg=0.61, what is the present cell state-of-charge (in percent)? Round to the nearest percent.
Answer = 75
Explaination: State of charge equals (theta(k) – theta(0))/(theta(100) – theta(0))
Q2. Suppose that a lithium-ion cell having total charge capacity Q=10Q=10Ah is discharged at a rate of 1A for 30min. What is the net change in the cell’s state of charge (in percent)? Round to the nearest percent. (Hint: I am looking for a signed answer — the answer is positive if the state of charge increases and it is negative if the state of charge decreases.)
Answer = -5
Explanation: The total charge removed is 0.5Ah, which is 1/20 of the cell’s capacity, or a decrease of 5%.
Q3. Consider the battery pack drawn below, where one cell has state-of-charge of 0% and the other cell has state-of-charge of 100%
Q3. Which of the following statements is most accurate regarding the battery pack’s state of charge?
- Battery pack state of charge should be the average of the two cell states of charge (i.e., 50%)
- Battery pack state of charge is ill-defined, and the term should never be used
- Battery pack state of charge is 0% because we cannot discharge the battery pack without damaging the cell having state of charge of 0%
- Battery pack state of charge is 100% because we cannot charge the cell having state of charge of 100%
Practice quiz for lesson 1.4.5 Quiz Answers
Q1. Consider a cell having nominal voltage v_{\rm nom}=3.3vnom=3.3V and total charge capacity Q=25Q=25Ah. If the BMS is designed to keep the cell’s state of charge between 5% and 95%, and if the cell’s present state of charge is 70%, what is the approximate remaining total energy in the cell (in Wh)? Round your answer to the nearest Wh.
- Ans=53.62=54
Q2. Consider the HPPC test depicted on slide 3 of this lesson for \Delta T=10ΔT=10s and I_{\rm chg}=I_{\rm dis}=10Ichg=Idis=10A. If the cell voltages at times {0, 10^++, 20^-−, 30^-−, 30^++, 40^-−, and 50} seconds are {3.8, 3.42, 3.4, 3.78, 4.15, 4.19, and 3.81}, what is the HPPC charge resistance R_{{\rm chg},\Delta T}Rchg,ΔT of this cell (in m\OmegaΩ)? Round your answer to the nearest m\OmegaΩ.
- Ans=While Discharge;40
Q3. Consider a cell having open-circuit voltage of 3.8V at its present state of charge, operational voltage limits of v_{\rm min}=2.5vmin=2.5V and v_{\rm max}=4.2vmax=4.2V, HPPC resistances of R_{{\rm chg},\Delta T}=0.01\OmegaRchg,ΔT=0.01Ω and R_{{\rm dis},\Delta T}=0.008\OmegaRdis,ΔT=0.008Ω. What amount of absolute discharge power (in W) does the HPPC method predict? Round your answer to the nearest W and report a positive number.
- Discharging Power;406
Practice quiz for lesson 1.4.6 Quiz Answers
Q1. A battery is built from four series-connected lithium-ion battery cells, where each cell has operational voltage limits v_{\rm min}=2.5vmin=2.5V and v_{\rm max}=4.2vmax=4.2V. If the present signed charge current limits of the four cells, computed via the HPPC approach, are {-85A, -81A, -83A, -82A}, what is the overall battery-pack absolute charge power capability (in W)? Round your answer to the nearest W and report a positive number.
- Discharge power capability (W)= 940
Q2. Consider a two-cell series-connected battery pack where cell states of charge are z_1=0.99z1=0.99 and z_2=0.97z2=0.97 and where the cells have total charge capacity Q_1=31Q1=31Ah and Q_2=32Q2=32Ah. If z_{\rm min}=0.1zmin=0.1, what is the maximum value for z_{\rm low}zlow (that is, \max_{j} (z_{{\rm low},j})maxj(zlow,j)) for this battery pack (in percent)? Round your answer to the nearest percent.
Answer:
To find the maximum value for �lowzlow, we need to consider the cell with the lowest state of charge (�low,�zlow,j) and calculate max�(�low,�)maxj(zlow,j) in percent.
In this case, �1=0.99z1=0.99 and �2=0.97z2=0.97. The minimum state of charge is �min=0.1zmin=0.1.
For cell 1: �low,1=�min−(1−�1)=0.1−(1−0.99)=0.1−0.01=0.09zlow,1=zmin−(1−z1)=0.1−(1−0.99)=0.1−0.01=0.09
For cell 2: �low,2=�min−(1−�2)=0.1−(1−0.97)=0.1−0.03=0.07zlow,2=zmin−(1−z2)=0.1−(1−0.97)=0.1−0.03=0.07
The maximum value for �lowzlow is max�(�low,�)=max(0.09,0.07)=0.09maxj(zlow,j)=max(0.09,0.07)=0.09 or 9% when rounded to the nearest percent.
Q3. Consider a two-cell series-connected battery pack where cell states of charge are z_1=0.99z1=0.99 and z_2=0.97z2=0.97 and where the cells have total charge capacity Q_1=31Q1=31Ah and Q_2=32Q2=32Ah. If z_{\rm min}=0.1zmin=0.1, and the cells can be considered to have constant OCV of 3.3V, what is the total battery discharge energy for this battery pack (in Wh)? Round your answer to the nearest Wh. (Note that this configuration is the same as in Question 2, which might save you some math.)
- Total battery discharge energy for the battery pack (Wh)= 182
Practice quiz for lesson 1.4.7 Quiz Answers
Q1. Which of the following are diagnostics that a BMS may need to compute and communicate to the host application or service technician? Select all that apply.
- A record of internal BMS failures detected
- A log of battery-pack abuse conditions caused by external factors
- The state of health of the weakest cell in the battery pack
- The efficiency of the thermal management system
Q2. Which of the following estimates attempts to predict how much life remains as either a percentage or amount of calendar time?
- State of power (SOP)
- State of Health (SOH)
- State of charge (SOC)
- State of Life (SOL)
Q3. Which of the following constitutes a BMS internal failure that should be logged if detected?
- Balancing-system failures
- Thermal-management-system failures
- Contactor failures
- Voltage, current, and/or temperature-sensor failures
- All of these options.
Introduction to battery-management Systems Week 05 Quiz Answers
Quiz for lesson 1.5.1
Q1. Consider a pouch cell built from five negative-electrode current collectors coated on both sides with negative-electrode active materials, and five positive-electrode current collectors coated on both sides with positive-electrode active materials. The cell is constructed with separator material between electrodes, and all negative-electrode current collectors are welded together and attached to the negative pouch-cell terminal, and all positive-electrode current collectors are welded together and attached to the positive pouch-cell terminal (as is standard).
How many negative-electrode/separator/positive-electrode cells are connected in parallel inside this pouch cell?
Answer: In a pouch cell built as described with five negative-electrode current collectors and five positive-electrode current collectors, the number of negative-electrode/separator/positive-electrode cells connected in parallel inside the pouch cell is 5. This means that there are five sets of negative-electrode/separator/positive-electrode layers connected in parallel within the cell.
Q2. Which of the following statements is true regarding the active materials used in the negative and positive electrodes of a lithium-ion cell?
- Negative-electrode active materials are green in color and positive-electrode active materials are purple in color
- Negative electrodes are usually built from a solid layer of graphite
- Electrode-active materials should be small particles that are as spherical as possible
- Positive electrodes are usually built from a solid layer of a lithium-metal-oxide material
Q3. The slurry that is coated onto the current collector foils comprises which of the following? Select all that apply.
- Conductive additives
- Solvent
- Electrode active materials
- Electrolyte
- Binders
Q4. What is electrode calendering?
- Compressing an electrode to a desired thickness
- Recording the electrode’s fabrication date for warrantee and diagnostics purposes
- Maintaining a log of electrode manufacture rate to ensure that cell production remains on schedule
- Inspecting the electrode coating to detect whether any particles are too small or too large
Q5. What is the correct order of operations for electrode fabrication?
1) Metal foils are unrolled
2) Slurry of electrode solid materials deposited on foils
3) The electrode is called
4) Dryers evaporate solvents
5) Foils are slit to desired final widths
1) Metal foils are unrolled
2) Foils are slit to desired final widths
3) Slurry of electrode solid materials deposited on foils
4) Dryers evaporate solvents
5) Foils re-reeled in preparation for next steps
1) Metal foils are unrolled
2) Slurry of electrode solid materials deposited on foils
3) Dryers evaporate solvents
4) The electrode is calendered
5) Foils are slit to desired final widths
1) Metal foils are unrolled
2) Slurry of electrode solid materials deposited on foils
3) The electrode is calendered
4) Dryers evaporate solvents
5) Foils re-reeled in preparation for next steps
Quiz for lesson 1.5.2 Quiz Answers
Q1. In a cylindrical or a prismatic cell, what is the primary reason we might desire to weld multiple tabs from each electrode’s current collector to its respective cell terminal?
- This enables the cell to deliver higher levels of power
- It is easier to build a cell having multiple tabs per current collector than it is to build a cell having only a single tab per current collector
- It gives the cell a more rigid structure
- It is less expensive to manufacture a cell having multiple tabs per current collector
Q2. Which of the following places steps performed during the cell assembly process in the correct order?
1) Building the electrode subassembly
2) Packaging the electrode subassembly into the cell casing
3) Filling the cell with electrolyte
4) Final inspection
5) Final sealing and welding
1) Building the electrode subassembly
2) Packaging the electrode subassembly into the cell casing
3) Filling the cell with electrolyte
4) Final sealing and welding
5) Final inspection
1) The formation process
2) Building the electrode subassembly
3) Packaging the electrode subassembly into the cell casing
4) Filling the cell with electrolyte
5) Final sealing and welding
1) Building the electrode subassembly
2) Filling the cell with electrolyte
3) Packaging the electrode subassembly into the cell casing
4) Final sealing and welding
6) The formation process
Q3. What is meant by the “formation” process?
- It is the process of charging the battery cell for the first time
- It is the overall process of fabricating a lithium-ion battery cell from beginning to end
- It is the process either of stacking electrodes (for pouch cells), or winding electrodes (for cylindrical or prismatic cells) for insertion into the cell’s package
- It is the remainder of the process of constructing a lithium-ion battery cell after the electrodes have already been fabricated
Q4. Why must the electrolyte-fill step be done in a dry room?
- It is not necessary to perform the electrolyte-fill step in a dry room
- Moisture absorbed by the electrolyte can lead to reaction products that prematurely degrade the cell
- It is more comfortable for the machine operators to work in a low-humidity environment
- Moisture in the environment reacts with the solvent in the electrolyte and can cause hazardous conditions inside the cell-fabrication plant
Q5. Which of the following problems can be directly detected during the formation process? Select all that apply.
- Abnormally high or low cell total charge capacities
- Out-of-tolerance levels of electrode contamination
- Unacceptably high rates of cell self-discharge
- Abnormally high or low cell impedance
Quiz for lesson 1.5.3 Quiz Answers
Q1. Which of the following are possible underlying causes of eventual cell failure over which a BMS may have some control? Select all that apply.
- Uncontrolled operations
- Cell design faults
- Poorly controlled cell manufacturing processes
- Normal aging
- Abuse
Q2. Which of the following aging processes is most accurately described as the consumption of the active chemicals in the cell through undesired chemical reactions with the environment?
- Dendritic growth
- Corrosion
- Crystal formation
- Passivation
Q3. Which of the following aging processes is most accurately described as the formation of treelike metallic structures on the electrodes?
- Dendritic growth
- Passivation
- Corrosion
- Evaporation
Q4. Which of the following aging processes is most accurately described as the growth of a solid–electrote interphase layer on the surface of electrode particles?
- Passivation
- Crystal formation
- Dendritic growth
- Corrosion
Q5. Which of the following is a likely set of consequences of cell aging?
- Cell total charge capacity decreases, cell impedance increases and cell self-discharge rate decreases
- Cell total charge capacity increases, cell impedance increases, and cell self-discharge rate decreases
- Cell total charge capacity decreases, cell impedance decreases, and cell self-discharge rate increases
- Cell total charge capacity decreases, cell impedance increases, and cell self-discharge rate increases
Quiz for lesson 1.5.4 Quiz Answers
Q1. What are some possible effects of violating cell temperature and/or voltage specifications? Select all that apply.
- Electrolyte breakdown
- Electrode plating
- Penetration of separator
- Gassing, swelling, and/or venting
- Overheating and thermal runaway
Q2. What are some possible types of physical abuse that might be experienced by a battery pack? Select all that apply.
- Being overcharged
- Being immersed in fluids
- Being dropped
- Being penetrated by a sharp object
- Being crushed
Q3. If one cell in a battery comprising series-connected cells fails to open the circuit, which of the following is always true?
- The battery will go into a thermal runaway
- The self-discharge rate of the remaining cells in the battery will quickly increase
- The battery still operates but at a reduced capacity due to the failed cell
- The battery is no longer able to supply electrical current to the load
Q4. If one cell in a battery comprising series-connected cells fails a short circuit, which of the following is always true?
- The battery is no longer able to supply electrical current to the load
- The battery will go into a thermal runaway
- The battery still operates but at a reduced capacity due to the failed cell
- The self-discharge rate of the remaining cells in the battery will quickly increase
Q5. Which of the following statements is true? Select all that apply.
- Many times, cell failure is directly caused by overheating
- Battery packs are expected to survive physical abuse
- If one cell in a battery pack has a “soft short circuit,” the battery can often still supply electrical current to the load
- Thermal runaway is when heat cannot be removed as quickly as it is spontaneously generated
Get All Course Quiz Answers of Algorithms for Battery Management Systems Specialization
Introduction to battery-management systems Coursera Quiz Answers
Equivalent Circuit Cell Model Simulation Coursera Quiz Answers
Battery State-of-Charge (SOC) Estimation Coursera Quiz Answers

